InVivoMAb anti-mouse CD4
| Clone | YTS 177 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalog # | BE0003-3 | ||||||||||||
| Category | InVivoMab Antibodies | ||||||||||||
| Price |
|
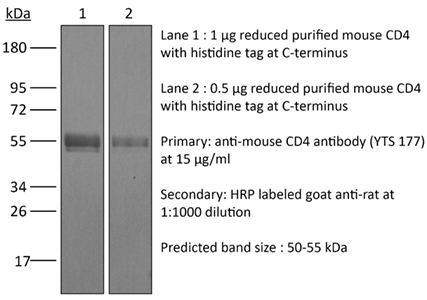
The YTS 177 monoclonal antibody reacts with the mouse CD4. The CD4 antigen is a 55 kDa cell surface type I membrane glycoprotein belonging to the immunoglobulin superfamily. CD4 acts as a co-receptor which in cooperation with the T cell receptor (TCR) interacts with class II MHC molecules displayed by antigen presenting cells (APC). CD4 is expressed by the majority of thymocytes, most helper T cells, a subset of NK-T cells and weakly by dendritic cells and macrophages. CD4 plays an important role in the development of T cells and is required for mature T cells to function optimally. The YTS 177 antibody has been shown to compete with clones GK1.5 and YTS 191 for CD4 binding. Additionally, the YTS 177 antibody has been reported to be non-depleting but binding does induce rapid internalization of CD4 on both CD4+ Foxp3- T cells and Foxp3+ regulatory T cells. Further, the YTS 177 clone has been shown to suppress or even prevent allograft rejection, allergic reactions and autoimmune responses.
| Isotype | Rat IgG2a |
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure™ pH 7.0 Dilution Buffer |
| Immunogen | Mouse Spleen Cells |
| Reported Applications |
|
| Formulation |
|
| Endotoxin |
|
| Purity |
|
| Sterility | 0.2 μM filtered |
| Production | Purified from tissue culture supernatant in an animal free facility |
| Purification | Protein G |
| RRID | AB_1107642 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
INVIVOMAB ANTI-MOUSE CD4 (CLONE: YTS 177)
Li, Z., et al. (2015). “Pre-treatment of allogeneic bone marrow recipients with the CXCR4 antagonist AMD3100 transiently enhances hematopoietic chimerism without promoting donor-specific skin allograft tolerance.” Transpl Immunol 33(2): 125-129. PubMed
Hematopoietic chimerism established by allogeneic bone marrow transplantation is known to promote donor-specific organ allograft tolerance; however, clinical application is limited by the need for toxic host conditioning and “megadoses” of donor bone marrow cells. A potential solution to this problem has been suggested by the observation that recipient bone marrow mobilization by the CXCR4 antagonist AMD3100 promotes chimerism in congenic bone marrow transplantation experiments in mice. Here we report that a single subcutaneous dose of 10mg/kg AMD3100 in recipient C57BL/6 mice was able to enhance hematopoietic chimerism when complete MHC-mismatched BALB/c donor bone marrow cells were transplanted 1h after drug dosing. However, levels of chimerism measured 30days post-transplantation were not sustained when mice were reexamined on day 90 post-transplantation. Moreover, transient chimerism induced by this protocol did not support robust donor-specific skin allograft tolerance. Using the same transient immunosuppression protocol, we confirmed that “megadoses” of donor bone marrow cells could induce durable chimerism associated with donor-specific skin allograft tolerance without AMD3100 pre-treatment. We conclude that in this protocol AMD3100 pretreatment may empty bone marrow niches that become reoccupied by allogeneic donor hematopoietic progenitor cells but not by true long-lived donor hematopoietic stem cells, resulting in short-lived chimerism and failure to support durable donor-specific allograft tolerance.
Mayer, C. T., et al. (2014). “Anti-CD4 treatment inhibits autoimmunity in scurfy mice through the attenuation of co-stimulatory signals.” J Autoimmun 50: 23-32. PubMed
A major concept in autoimmunity is that disruption of Foxp3(+) regulatory T cells (Tregs) predisposes to breach of tolerance. This is exemplified by the Foxp3-linked disorder termed IPEX (immunodysregulation, polyendocrinopathy, enteropathy, X-linked) which affects newborn children. There has been considerable clinical interest in the role of non-depleting anti-CD4 antibodies as a means of upregulating the function of Foxp3(+) Tregs in order to control detrimental inflammatory responses such as transplant rejection. However, according to the paradigm of a Treg-dependent mechanism of action, the effectiveness of anti-CD4 antibodies as a therapy for human autoimmune diseases is unclear considering that Treg function might be intrinsically impaired. Specifically, anti-CD4 therapy is expected to fail in patients suffering from the IPEX syndrome due to the lack of functional Foxp3(+) Tregs. Taking advantage of natural Foxp3 mutant scurfy (sf) mice closely resembling the IPEX syndrome, and genetically engineered mice depleted of Foxp3(+) Tregs, we report here that anti-CD4 treatment induces tolerance independent of Foxp3(+) Tregs. This so far undefined mechanism is dependent on the recessive non-infectious tolerization of autoreactive T cells. Treg-independent tolerance alone is powerful enough to suppress both the onset and severity of autoimmunity and reduces clinically relevant autoantibody levels and liver fibrosis. Mechanistically, tolerance induction requires the concomitant activation of autoreactive T cells and is associated with the down-regulation of the co-stimulatory TNF-receptor superfamily members OX40 and CD30 sustaining CD4(+) T cell survival. In the light of ongoing clinical trials, our results highlight an unexpected potency of anti-CD4 antibodies for the treatment of autoimmune diseases. Particularly, CD4 blockade might represent a novel therapeutic option for the human IPEX syndrome.
Rocca, C. J., et al. (2014). “rAAV9 combined with renal vein injection is optimal for kidney-targeted gene delivery: conclusion of a comparative study.” Gene Ther 21(6): 618-628. PubMed
Effective gene therapy strategies for the treatment of kidney disorders remain elusive. We report an optimized kidney-targeted gene delivery strategy using recombinant adeno-associated virus (rAAV) administered via retrograde renal vein injection in mice. Renal vein injection of rAAV consistently resulted in superior kidney transduction compared with tail vein injection using as little as half the tail vein dose. We compared rAAV5, 6, 8 and 9, containing either green fluorescent protein (GFP) or luciferase reporter genes driven by the Cytomegalovirus promoter. We demonstrated that although rAAV6 and 8 injected via renal vein transduced the kidney, transgene expression was mainly restricted to the medulla. Transgene expression was systematically low after rAAV5 injection, attributed to T-cell immune response, which could be overcome by transient immunosuppression. However, rAAV9 was the only serotype that permitted high-transduction efficiency of both the cortex and medulla. Moreover, both the glomeruli and tubules were targeted, with a higher efficiency within the glomeruli. To improve the specificity of kidney-targeted gene delivery with rAAV9, we used the parathyroid hormone receptor ‘kidney-specific’ promoter. We obtained a more efficient transgene expression within the kidney, and a significant reduction in other tissues. Our work represents the first comprehensive and clinically relevant study for kidney gene delivery.